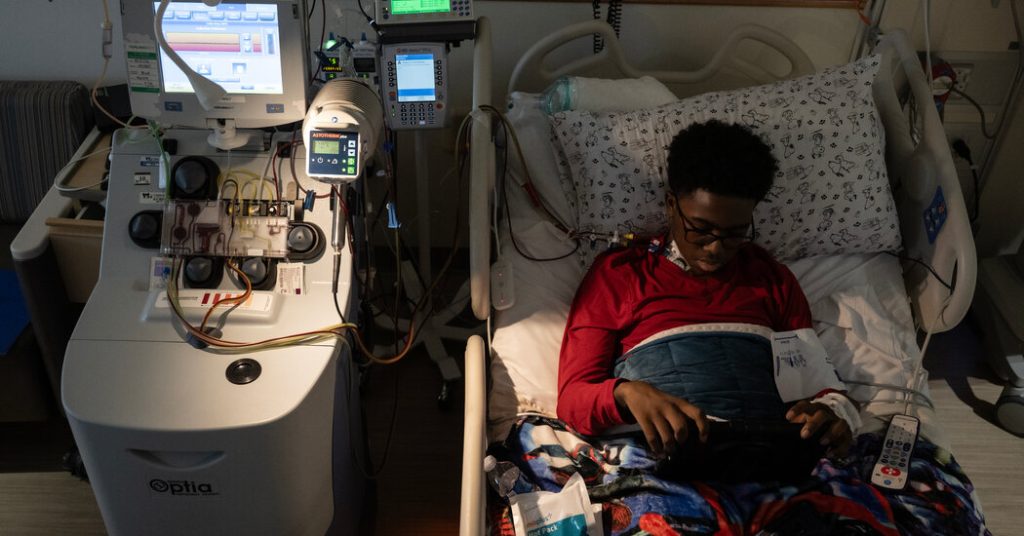
Kendric Cromer, a 12-year-old boy, has made headlines as the first patient to undergo a recently approved gene therapy for sickle cell disease, having left the Children’s National Hospital in Washington, D.C., after 44 days of intensive treatment. As reported by the New York Times, this groundbreaking moment marks a significant milestone in the field of genetic medicine, striking a note of hope for individuals affected by a condition that impacts around 100,000 people in the United States. Sickle cell disease is chiefly caused by a genetic mutation in hemoglobin, leading to a host of painful symptoms and complications that can severely affect one’s quality of life.
Until late 2023, treatment options for sickle cell disease were limited and often inadequate, offering little solace to those grappling with the condition. However, a turning point arrived with the U.S. Food and Drug Administration’s approval of two revolutionary gene therapies: Bluebird Bio’s treatment priced at $3.1 million, and a $2.2 million therapy from Vertex Pharmaceuticals. These innovative approaches represent a significant advancement in medical science, as they provide the potential for a permanent correction of the genetic mutation responsible for the disease. For many, this breakthrough offers a tantalizing glimpse into a future free from the lifelong struggles associated with sickle cell disease.
Kendric’s journey has not been without its emotional and physical challenges. Reflecting on his experiences, he expressed a deep sense of resignation prior to receiving treatment, believing that sickle cell was a condition he would endure forever. His sentiments resonate with the broader narrative of those suffering from the disease, who often feel that their lives are unfairly constrained by their health issues. The challenges of growing up with a chronic illness can be profound, significantly affecting one’s childhood and overall well-being. For Kendric, undergoing gene therapy was a brave step toward reclaiming the life that the disease had once overshadowed.
The decision to pursue gene therapy was not an easy one for Kendric’s family. His parents revealed that despite extensive consultations with medical professionals and their own diligent research into the complexities of sickle cell disease, they found themselves unprepared for the emotional and physical toll the treatment process would exact on their son. They had anticipated difficulties, but the reality of the experience turned out to be more challenging than they could have ever imagined. Their narrative illustrates the often-overlooked emotional struggle that families face while navigating treatment decisions for chronic illnesses.
The transformative potential of these gene therapies cannot be understated. With each passing day, patients like Kendric are becoming symbols of hope that not only convey the power of modern medicine but also inspire a renewed sense of optimism for those affected by sickle cell disease. For many families, the approval of these therapies opens the door to a future where their loved ones may live free from the constant burden of pain and medical complications. The prospect of alleviating suffering through advanced genetic treatments offers a compelling reason for hope amid the struggles associated with managing sickle cell disease.
Ultimately, the case of Kendric Cromer reflects the intersection of hope, adversity, and scientific advancement in the realm of healthcare. His journey has highlighted both the challenges faced during treatment and the immense possibilities that new therapies can offer. As families continue to grapple with the realities of chronic illnesses like sickle cell disease, Kendric’s story stands as a testament to the courage and resilience of young patients and their families, emboldened by the prospect of a healthier future through groundbreaking medical innovations. The approval of gene therapies represents not just a medical achievement, but a beacon of hope for all those affected by this challenging condition.